 JE-QP-ISO753 - Control of Documents Policy
JE-QP-ISO753 - Control of Documents Policy
| Doc #: JE-QP-ISO753 | Title: Control of Documents Policy | Print Date: XX/XX/XX |
| Rev: 1.00 | Prepared By: Joseph J Gillis/CEO | Prepared On: 12/29/2025 |
| Effective: 01/01/2026 | Reviewed By: Joseph J Gillis/CEO | Reviewed On: 12/29/2025 |
| ISO 9001:2015 | Approved By: Joseph J Gillis/CEO | Approved On: 12/29/2025 |
| Policy: | Our organization maintains a systematic process to identify, review, and approve documented information to ensure it remains accurate, legible, and available where needed while preventing the unintended use of obsolete documents ISO 9001:2015 Clause 7.5. | ||||||||||||
| Purpose: | This document defines methods and responsibilities for controlling quality-related internal and external documents. It establishes procedures for document approval, revision, and distribution. | ||||||||||||
| Scope: | This policy applies to all controlled documents including:
|
||||||||||||
| Responsibilities: | Quality Assurance Department
Department Managers
|
||||||||||||
| Definitions: | Internal Document: Document created within the organization., e.g. policies, working instructions, records etc.
External Document: Document originating from outside the organization., e.g. data sheets, government forms, warranties etc. Controlled Document: Document that provides information or direction necessary for provision of product or service that is governed by the organization's Quality Management System (QMS). Document Control Number (DCN): A document specific identification number assigned to controlled documents in accordance with the organization's Document Numbering System (Appendix A). |
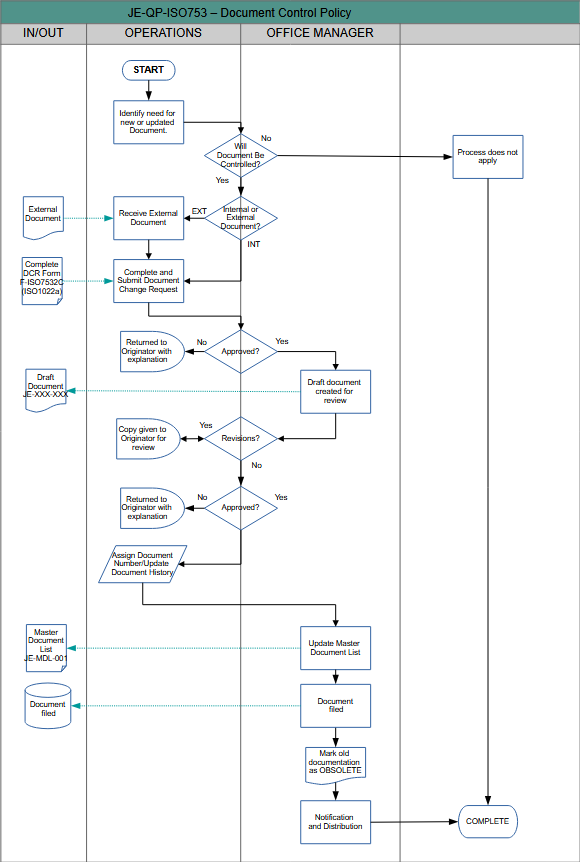
Procedure:
- The need for a new or updated document is identified:
- Submissions can be made through completions of JE-F-XXX - Document Change Request Form (ISO7532C).
- Each request will be reviewed for approval by the Quality Assurance Department prior to release for advancement.
- Should the request be rejected, the form will be returned to the originator with an explanation.
- A draft document is created in accordance with the organization's Documentation Standards (Appendix C).
- The document is reviewed by Management
- If changes are deemed necessary, notes from Management are attached to the document and it is returned to the originator to correct.
- If the document is rejected or deemed unnecessary by Management at this time, originator will be notified.
- Documents approved by Management will be signed off and forwarded to the Quality Manager.
- The Quality Assurance Department will review the submitted document for compliance.
- Final approval of the document is provided by the President/CEO or designated authority.
- For new documents Quality Assurance will assign a Document Control Number (DCN) in accordance with the organization's Document Numbering system (Appendix A).
- The version number and revision date are updated in the document history table.
- The Master Document List (See: JE-MDL-001 - Master Document List) is updated with the new information.
- Obsolete documentation is tagged and achieved
- Document is published/released
- Only approved documents can be published externally.
- Current document distribution list is maintained/updated.
- Distribute approved documents to authorized personnel.
- Ensure controlled access through documentation of distribution activities.
- Notify personnel of document revisions.
- Retrieve obsolete documents deemed confidential to archive and/or destroy.
- Electronic documents are available to authorized personnel through company username and password.
- Electronic availability is subject to document access control and security restrictions that may have been placed on the document.
- Control of all electronic documents related to quality will be established through user or group specific server permissions maintained by the IT Administrator.
Document Storage and Retention:
- Locations storing published company documents are to be continuously monitored - either physically or electronically through personal or security devices.
- Backup copies of electronic documents are performed nightly (incremental) and weekly (full).
- Unless noted, QMS documentation will be retained indefinitely.
- Physical offices are to be securely locked after hours for security purposes.
- Document accessibility shall be periodically verified.
Auditing of this policy:
- Audits of the Document Control System will be conducted annually to:
- Verify document availability and accuracy.
- Ensure compliance with this policy.
- Audit findings and corrective actions are to be recorded in the Auditing Review History section of this document.
References – Listing of references cited in this Procedure:
- Document Numbering System (Appendix A)
- Electronic Document Storage System (Appendix B)
- Document Standards (Appendix C)
- Procedure Flow Diagram (Appendix D)
- Policy/Procedure Form Header (Appendix E)
- Document History and Version Control (Appendix F)
Documents – Listing of documents cited in this procedure:
- ISO7532C – Document Change Request Form
- MDL-001 – Master Document List
APPENDIX A – DOCUMENT NUMBERING SYSTEM (DNS)
Our organization uses the following numbering system to uniquely identify internally controlled documents:
|
ISO Sited Policies |
QP-ISOXxx where X corresponds to the related ISO 9001:2015 section and xx relates to the sub-section number for policies related to specific ISO 9001:2015 needs |
|
ISO Sited Procedures |
PRO-ISOXxx where X corresponds to the related ISO 9001:2015 section and xx relates to the sub-section number for policies related to specific ISO 9001:2015 needs |
|
ISO Sited Forms |
F-ISOXxx where X corresponds to the related ISO 9001:2015 section and xx relates to the sub-section number for forms related to specific ISO 9001:2015 needs |
|
Policies |
POL-XXX where XXX is a sequential number starting with 001 for interoffice policies. |
|
Procedures |
ZZZ-PRO-XXX where ZZZ is the organizational number and XXX is a sequential number starting with 001 for interoffice procedures. |
|
Forms |
ZZZ-F-XXX where ZZZ is the organizational number and XXX is a sequential number starting with 001 for controlled QMS forms. |
|
Master Document Lists |
MDL-XXX where XXX is a sequential number starting with 001 for each independent document list |
|
Proposal (CRM) |
PRO(P)-XXXX - where XXX is a sequential number starting with 001 for each Proposal Assigned by CRM |
|
Estimate (CRM) |
EST-XXXX - where XXX is a sequential number starting with 001 for each Proposal Assigned by CRM |
APPENDIX B – ELECTRONIC DOCUMENT STORAGE FOLDERS
|
Description |
Location on Server |
|
Master Document Lists |
|
|
Obsolete Documents |
|
|
Quality Policies |
|
|
Procedures |
|
|
Forms |
|
|
Proposals |
|
|
Estimates |
|
|
Management Reviews |
|
|
QMS Audit results |
APPENDIX C – DOCUMENT STANDARDS
Standard Format
The standard format for all QMS controlled documents shall include:
-
Header containing company Logo
-
Footer containing document number, document name, revision number
-
A document history and version control listed on the last page (Appendix F). This does not apply to completed Records.
The standard format for QMS Policy/Procedure shall include:
-
Header containing company logo, document name
-
Footer containing document number, document name, revision number, page number
-
A heading table on the first page containing: document number, revision number, effective date, document title, who the document was prepared by, reviewed by, and approved by, the date that the document was prepared, reviewed, and approved. (Appendix E)
-
Policy (mission or standard that the procedure must meet)
-
Purpose/Objectives (what is the rationale of this procedure)
-
Scope (what areas of the company are affected by this procedure)
-
Responsibilities (who is involved in the procedure and what they are required to do)
-
Definitions (any words that are used that could be unclear to the reader)
-
Procedure
-
Related documents (such as procedure flow charts or referenced forms)
-
A document history and version control listed on the last page
APPENDIX D – DOCUMENT FLOW
APPENDIX E – EXAMPLE OF POLICY/PROCEDURE FORM HEADER
| Doc #: | Title: | Print Date: |
| Rev: | Prepared By: | Prepared On: |
| Effective: | Reviewed By: | Reviewed On: |
| ISO 9001:20xx | Approved By: | Approved On: |
Annual Review History
This policy will be reviewed annually and updated as necessary to maintain effectiveness and compliance with quality standards.
THE FOLLOWING FORM IS TO BE COMPLETED DURING ANNUAL AUDIT
| Date | Reviewed by | Notes |
Document Version History
| Version | Reviewer | Approver | Approval Date | Description |
| 1.0 | JG | JG | 2025-12-31 | Creation of Document |